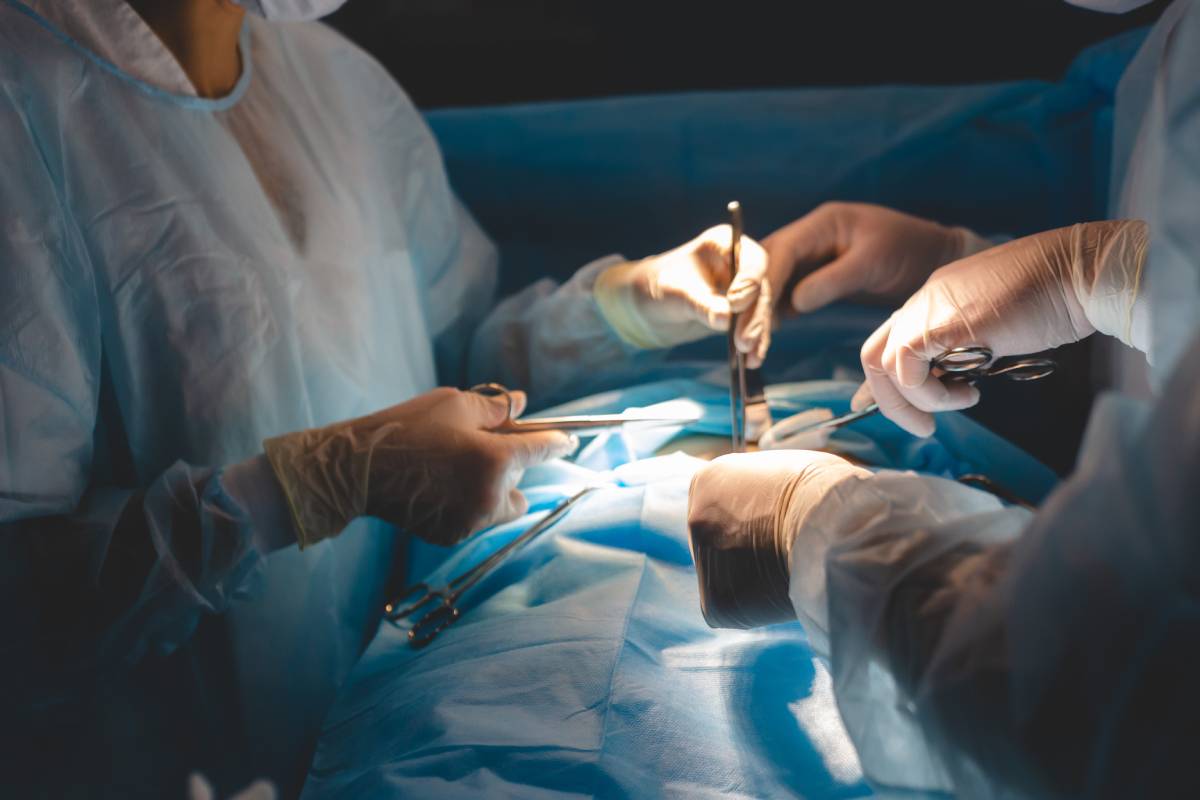
Surgery in the prone position, or lying face down, as opposed to supine where the patient is lying on their back with their face up, is used when a surgery requires access to anatomical structures posteriorly. This can include access to the posterior head, neck, or spine during a spinal surgery or even for access to the upper urinary tracts during urological surgeries [1].
Prone surgery is associated with many complications that often stem from the prolonged and increased pressure on anterior organs. Increased abdominal pressure causes excess pressure to build on the inferior vena cava, the major vein which returns blood from the body back to the heart. This results in a back-up of blood in the body and thus, less blood returning to the heart. Further, the pressure on the chest wall leads to decreased output from the heart which results in a subsequent drop in blood pressure. This drop in blood pressure can be dangerous in the setting of surgery. Additionally, respiratory rate is affected in prone surgery, which can lead to further decreases in cardiac output and oxygenation [2]. Some of the potential cardiovascular complications of prone surgery include hypovolemia and cardiac arrest [1]. Increased bleeding is also a common complication of the prone position, especially in spinal surgery, as damage to engorged veins can cause greater bleeding than damage to normal veins [2].
Prone positioning can also increase intraocular pressure [3]. Malpositioning of the patient can lead to direct pressure on the eye for an extended period, which can lead to irreversible visual loss. While postoperative vision loss is commonly described in journals, the true rate of incidence remains low [1]. However, while rare, it is necessary for both surgeons and anesthesiologists to watch for adequate patient eye protection during prone surgeries to avoid this potentially devastating complication.
There are many preoperative risk factors that play a role in postoperative complications of prone surgery. In terms of postoperative vision loss, older patients who have an elevated intraocular pressure at baseline typically are at higher risk for vision loss [3]. Hypertension, diabetes, obesity, anemia, atherosclerosis, and history of smoking are all risk factors for postoperative complications [5].
Ideally in a prone surgery, positioning will maximize ventilation of the patient while minimizing risk of bleeding and damage to vital organs [2]. Over the years, special operating tables and other devices such as chest rolls and bolsters have been designed to promote ideal prone positioning, decrease pressure on the abdomen and chest, and lower complications associated with prone positioning. Further, careful positioning can prevent vision loss by changing the degree of the bed so that the head is above the heart. This allows for less venous pooling of blood in the eye and orbit which can help to decrease intraocular pressures [4]. Reducing the length of time patients are in prone positioning can also help to decrease complications. Surgeries that are longer than 6.5 hours are considered prolonged, and it may be beneficial to use a series of shorter surgeries to reduce risks of complications. On the other hand, risks of multiple surgeries may outweigh the risks of one prolonged prone surgery [3,5]. Overall, for preventing complications of prone surgery, it is important to monitor fluids, using IV fluids when necessary to replace intravascular volume, monitor blood loss and hemoglobin level, and optimize blood pressure within 20-25% of baseline blood pressure [5,6].
References
- Kwee, Melissa M., et al. “The Prone Position during Surgery and Its Complications: A Systematic Review and Evidence-Based Guidelines.” International Surgery, vol. 100, no. 2, Feb. 2015, pp. 292–303, www.ncbi.nlm.nih.gov/pmc/articles/PMC4337445/, 10.9738/intsurg-d-13-00256.1.
- Schonauer, Claudio, et al. “Positioning on Surgical Table.” European Spine Journal, vol. 13, no. S01, 22 June 2004, pp. S50–S55, 10.1007/s00586-004-0728-y.
- van Wicklin, Sharon Ann. “Systematic Review and Meta-Analysis of Prone Position on Intraocular Pressure in Adults Undergoing Surgery.” International Journal of Spine Surgery, 14 Apr. 2020, p. 7029, 10.14444/7029.
- Ozcan, Mehmet S., et al. “The Effect of Body Inclination during Prone Positioning on Intraocular Pressure in Awake Volunteers: A Comparison of Two Operating Tables.” Anesthesia & Analgesia, vol. 99, no. 4, Oct. 2004, pp. 1152–1158, 10.1213/01.ane.0000130851.37039.50.
- Shifa, Jemal, et al. “A Case of Bilateral Visual Loss after Spinal Cord Surgery.” Pan African Medical Journal, vol. 23, 2016, 10.11604/pamj.2016.23.119.8443.
- Lee, Lorri A., et al. “The American Society of Anesthesiologists Postoperative Visual Loss Registry.” Anesthesiology, vol. 105, no. 4, 1 Oct. 2006, pp. 652–659, 10.1097/00000542-200610000-00007.